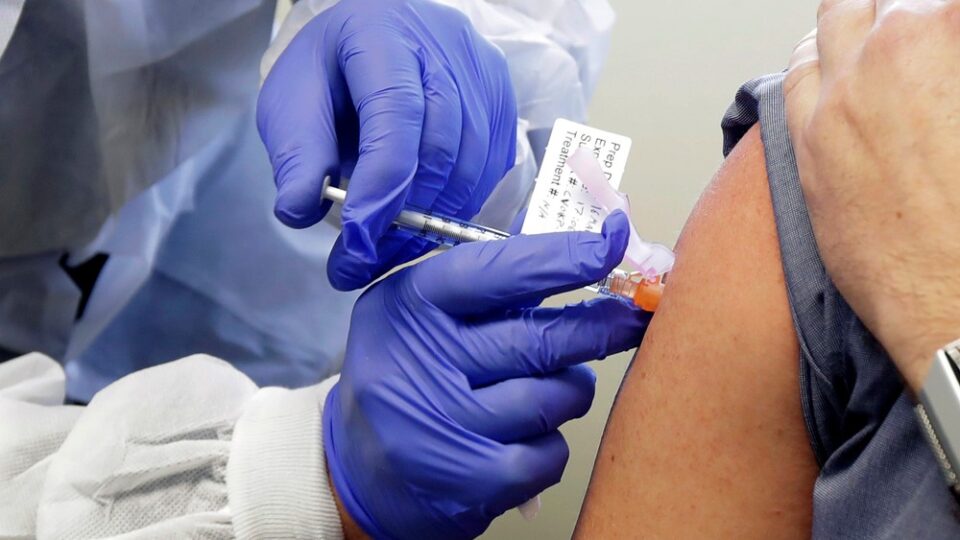
The first coronary vaccine may be accepted for use in the EU and Norway one week earlier than planned. Thus, the first can be vaccinated in Norway during the Christmas holidays.
It has previously been stated that the first coronary vaccine is likely to be recommended for use in the EU and Norway shortly after an advisory meeting on 29 December.
It now appears that the first vaccine may arrive in Norway shortly after 21 December. According to NRK, the European Medicines Agency (EMA) will expedite the advisory meeting to that date.
According to what NRK is informed, it is Germany that has pressed for the meeting to be accelerated, which will provide advice on approval. Bildt Zeitung reports that a conditional approval may come on 23 December.
It is the vaccine for Biontech / Pfizer that is closest to receiving limited approval in the EU. FHI has previously said that it is possible to get doses of this to Norway in January.
To christmas
According to NRK, this may mean that vaccination can begin in Norway as early as Christmas Eve. But Minister of Health Bent Høie thinks this is somewhat optimistic.
– It is not very likely that it can happen before Christmas, but it can happen faster than we assumed just a short time ago. This has not been confirmed yet, and it is something we stay up to date on, says Høie.
– We are dependent on getting the vaccine to Norway after approval. Only then can we start the vaccination, but it will be to a small extent in the first place, he explains to NRK.
The vaccine ready
Large quantities of the vaccine have already been produced in Europe. According to what NRK is informed, it is only a matter of time it takes to send the first doses to Norway that determines when vaccination can begin.
– We have already pre-produced vaccine for the EU. As soon as we get the green light from the EMA and the European Commission, we can start loading and delivering the vaccine doses to the vaccination centers, a spokeswoman from the company Biontech told Bild Zeitung.
The system in Norway must be ready to receive the vaccine and get them into the arm of the first.
It is expected that the EU will give a conditional approval shortly after the vaccine meeting. According to information from Bildt Zeitung, it could happen on Christmas Eve.
That is, if the EU is advised to use the vaccine. It’s not safe.